Abstract
Introduction Whole body low-dose computed tomography (WBLDCT) is standard of care in the diagnostic work-up of suspected multiple myeloma. While the focus of clinical routine evaluation lies on the detection of osteolytic lesions or extramedullary myeloma manifestations, the entire informative capacity of WBLDCT scans seems underestimated. Here, the analysis of non-focal signal alterations might provide further diagnostic insights, and a recent study by Terao et al. described tumor-burden dependent loss of the splenic signal in diffusion-weighted MRI in newly diagnosed myeloma (NDMM) patients. However, due to limited availability of MRI, most NDMM patients receive WBLDCT at initial diagnostic work-up. Therefore, we asked if non-focal signal alterations of the spleen in WBLDCT scans (i.e., radiographic tissue density of the organ) in NDMM patients might correlate with disease characteristics, cytogenetics and clinical outcomes.
Methods In this retrospective analysis, we screened clinical records of NDMM patients diagnosed and treated at our institution (LMU University Hospital Munich, Germany) between 01/2010 and 12/2020. Clinical and laboratory data from initial diagnosis until first relapse were integrated with segmentation analyses of WBLDCT scans taken within 90 days before diagnosis. Plasma cell dyscrasias others than MM as well as cases with CT scans taken >90 days before diagnosis or CT scans containing contrast media were excluded.
Results A total of 231 consecutively treated NDMM patients were included with a median age of 66 years (range 25-89). Cytogenetic analyses were available in 177 (77%) patients of which 38 had high-risk (HR) aberrations including t(4;14), t(14;16) and del(17p). In 44 (19%) of patients a gain(1q) was found. Patients were classified according to ISS and R-ISS: ISS-1 84 (43%), ISS-2 56 (28%), ISS-3 57 (29%) and R-ISS-1 38 (22%), R-ISS-2 119 (70%), R-ISS-3 13 (8%). Induction therapy consisted of a doublet regimen in 61 (26%), a triplet regimen in 167 (72%) and a quadruplet regimen in 3 (1%) patients. From the entire cohort, 141 (61%) patients received high-dose melphalan and autologous stem cell transplantation (auto-SCT), and 46 (20%) patients underwent tandem auto-SCT. Maintenance therapy after auto-SCT was applied to 49 (35% of auto-SCT) patients.
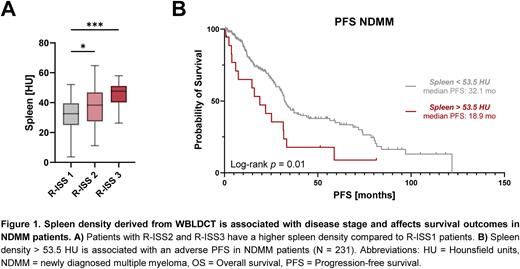
Spleen density gradually increased with ISS and R-ISS stages. Thus, ISS3 and R-ISS3 displayed a significantly increased spleen density compared to ISS1 and R-ISS1 patients (ISS3 40.7 HU vs. ISS1 35 HU, p=0.003; R-ISS3 45.4 vs. R-ISS1 32.5 HU, p=0.004, Fig. 1A). We further found spleen density to be significantly higher in patients with HR cytogenetics (HR vs SR HU, p=0.03), in particular with t(4;14) (p=0.04), but not with del(17p), t(14;16) or gain(1q). Moreover, it positively correlated with serum lambda light chain levels in patients with lambda paraproteinemia (r = 0.26, p = 0.02), but not with kappa light chain or immunoglobulin levels.
Based on an optimal log-rank test separation algorithm, NDMM patients with a spleen density of > 53.5 HU (Spleenhigh) had a shorter survival compared to Spleenlow patients (median PFS: 18.9 vs 32.1 months, p=0.01, Fig. 1B; median OS: not reached, p=0.02). PFS and OS differences were similar in subgroup analysis of transplant-eligible (PFS: p=0.08; OS: p=0.04) and transplant-ineligible patients (PFS: p=0.02, OS: p=0.04). In multivariate cox regression analyses including Spleenhigh/low and R-ISS stages, spleen density remained an independent factor for shorter survival (HRPFS 2.45, 95%CI 1.21-4.49, p=0.007; HROS 3.24, 95%CI 1.06-8.23, p=0.02).
Conclusion Our data suggest that high density of the splenic tissue in WBLDCT is associated with high-risk features and inferior survival of NDMM patients. Increased cell numbers or deposition of light chains within the spleen might represent modes of action. If validated in future prospective studies, spleen WBLDCT density may complement existing risk-stratification tools.
Disclosures
Rejeski:Kite/Gilead: Other: Travel Support, Research Funding; Novartis: Honoraria. Subklewe:Miltenyi Biotech: Research Funding; Morphosys: Research Funding; Roche: Consultancy, Research Funding; Seagen: Research Funding; Takeda: Other: Travel support; Pfizer: Consultancy; Novartis: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Gilead: Consultancy, Research Funding, Speakers Bureau; Celgene/BMS: Consultancy, Speakers Bureau; Amgen: Consultancy, Research Funding, Speakers Bureau; Seattle Genetics: Research Funding; Bristol-Myers Squibb: Research Funding. Theurich:Janssen: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; GSK: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.